Acrylamide
Acrylamide is the common name for 2-propenamide (Acrylic amide) chemical formula C3H5NO.
Acrylamide is a product used in the plastics industry, or which may appear spontaneously when cooking foods to more of 120 ° C.
It is a carcinogenic molecule and reprotoxic *. (*the animal, not studied in humans) which is considered by the who as posing a risk to human health. It is formed when cooking (frying, roasting...) high temperature of foods carbohydrate (starch, sugars) and protein.
Summary
1 Acrylamide and food
2 Concentration in the food
3 Effects on humans
4 Effects on the environment
4.1 Avoid the most acrylamide formation
5 Use in science
6 Toxicology
7 Method of analysis
Acrylamide and food
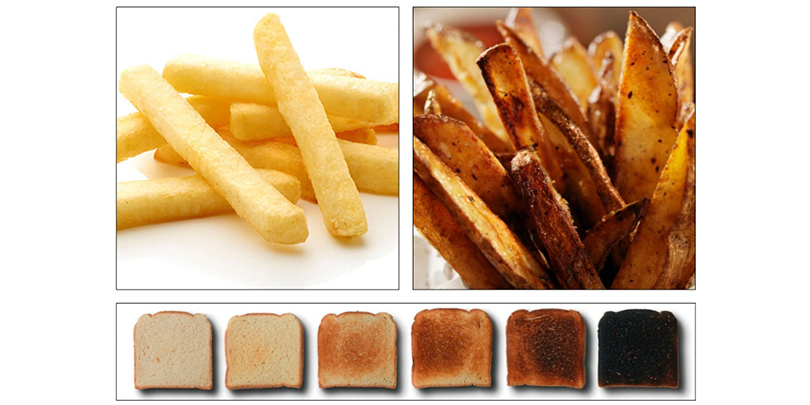
The formation of acrylamide is strongly influenced by the cooking temperature, the moisture content of food, as well as the 'Browning '. (carbonization) products.
The substance is synthesized especially when asparagine, a natural amino acid, between reacts with natural sugars such as glucose, It is the Maillard reaction, the name of the french chemist who identified for the first time. It is precisely this reaction which gives fried foods their taste, their consistency and their brown color so characteristic.
Most concerned food by this training are primarily based on cereals and potato products (such as chips or French fries), breads and pastries and generally all products subject to high temperatures such as coffee and toasted almonds.
Concentration in the food
Daily consumption of food contaminated by acrylamide is estimated at 0,001 mg/kg weight/day for the general population and to 0,004 mg/kg weight/day for large consumers.
By lowering the pH in the corn chips or French fries by the addition of citric acid before putting in the oven, the level of acrylamide is reduced from up to 80%. Solution of citric acid or acetic acid comes to extract the precursors of the potato.
Concentration of acrylamide in food 2002-2004 Food Concentration (µg/kg)
Chips 752
Coffee 509
Bread 446
Biscuits 350
French fries 334
The 306
Potato baked 169
Dried fruits 131
Cereals 96
Condiments and sauces 71
Fresh vegetables 59
Nuts and grains 51
Seafood 25
Meat 19
Boiled potato 16
Paste boiled 15
Alcoholic beverages 6.6
Milk and milk products 5.8
Boiled vegetables 4.2
Fresh fruit 0.8
Effects on humans
In 2002, While exposure data missing, the European Commission has started the collection of data of occurrence of acrylamide levels in food, then EFSA task in 2006, in collaboration with the Member States. The substance is considered to be effects on the nervous system, causing damage to the peripheral nervous system.
A study, funded by the EU showed a positive association between a high acrylamide levels in the blood and the development of breast cancer. The authors point to the fact that their study does not prove the existence of a direct link between this acrylamide in food and cancer but however evoke this possibility which must be confirmed by further work.
Effects on the environment
Acrylamide is biodegradable and accumulates in the environment or in the food chain. Some microorganisms are capable of degrading anaerobic or aerobic manner acrylamide; with or without light. The Rhodococcus genus for example, Converts the acrylamide acrylic acid monomer, a less toxic substance. However, significant degradation requires a minimum of a few days. The complete degradation of acrylamide can take days, weeks or months depending on the quantity of microorganisms in aqueous medium. Under aerobic conditions, the half-life of acrylamide in the rivers is 55 to 70 hours, slightly shorter than in sea water or estuary.
In several countries, to maintain a limit of 0.25 ?g/L of acrylamide in drinking water, the monomer concentration of acrylamide in polyacrylamide used for treatment of water is limited to 0.05% (0.5 g/kg). If the polyacrylamide is used for food packaging, the concentration is limited to 0.2% (2 g/kg).
Avoid the most acrylamide formation
An adult would consume on average 0,4 ?g (millionth of a gram) of acrylamide per kilogram of body weight each day. Without certainties, Researchers advise however reduce the amount ingested.
For this, It is recommended that :
- reduce cooking time, major parity of acrylamide formed in effect during the last minutes of cooking ;
- avoid consuming food charred or fried or roasted at temperatures equal to or greater than 120 ° C ;
- prefer big and thick foods that contain proportionately less acrylamide (but requiring longer cooking to be baked in heart) ;
- egg yolk would reduce acrylamide formation in a recipe.
For potatoes :
- Choose a variety of potato that contains less asparagine and sugars ;
- do not keep them in the refrigerator because this promotes the synthesis of precursor of the Maillard reaction ;
- the nitrogen-based fertilizers are also generators of precursors of the Maillard reaction ;
- do not eat potatoes that have sprouted or have become greenish.
The CIAA (Confederation of European agrifood industries) has published and periodically updates a 'Acrylamide Toolbox '., from the knowledge available, to help the food industry to voluntarily reduce food acrylamide levels.
Use in science
In molecular biology, using polymerized acrylamide (polyacrylamide) for electrophoresis. In polymer chemistry, It can be used as monomer participating in the formation of cross-linked polymers (or even gels crosslinked employees protocols).
Toxicology
Toxique “environnemental”
Acrylamide has first been identified as highly reactive industrial chemical toxic, notably present in tobacco smoke. The neurotoxicity of acrylamide in humans revealed via accidental exposures or professional to high rates of this product. Acrylamide is also a carcinogen and known reprotoxic (on laboratory animals).
Toxic food :
These are Swedish researchers who in 2002 have the first revealed its presence in food.
The former Scientific Committee on food (SCF) has issued an opinion on acrylamide in 2002, then a first study was published on acrylamide in foods. Other studies have been funded by the EU.
Acrylamide is one of risk products studied by the Scientific Panel on contaminants in the food chain (CONTAM) of the European food safety authority (EFSA), After a report of the Joint FAO/who food additives expert.
Biochemical aspect :
Acrylamide is rapidly absorbed and distributed in the body of the animal, and concentrated in the blood with a half life of two hours. Acrylamide is biotransformed by conjugation to glutathione before its degradation. Metabolites excreted in the urine are not toxic and represent 90% Acrylamide absorbed. In 2004, a study showed that about two hours after eating of chips containing potato 938 ± 1 µg of acrylamide, 4.5% Acrylamide is found in the urine (study on three men and three women).
Method of analysis
The formation of acrylamide in food is not distributed homogeneously but largely surface. For this reason, the portion of food must be homogenized before sample extraction and analysis. Most methods use isotopic acrylamide, 2H3 - AA or 13 c acrylamide-AA, as an internal standard. The internal standard is added before extraction.
Extraction of acrylamide in cold water in a ratio of 1:10 is very fast in a few minutes for hydrophilic samples such as cereals, potatoes, etc. If the sample contains a fat matrix (chocolate, peanut butter, etc), need to extract it with a non-polar solvent to remove the phase lipid.
The organic solvent extraction is an alternative method to extract water. Acetonitrile, methanol, propanol, the blend of ethanol/dichloromethane, etc are examples of polar organic solvents that have been used to extract food acrylamide. However, a hydration of food is necessary to ensure efficient retrieval.
Acrylamide is derived in 2, 3-dibromopropionamide for best sensitivity. The bromination of acrylamide occurs at 5 ° C for one night. The bromine derivative is extracted in ethyl acetate and dried with sodium sulfate and then concentrated by evaporation of the solvent.
GC - MS analysis directly without deriving gave higher scores because the acrylamide formed when parsing. Additional acrylamide formation is caused by the presence of the precursor of acrylamide. The analysis by GC - MS with derivation can give a LOQ until 5 µg/kg.
Acrylamide is a low UV chromophore. LC - UV is ideal for the analysis of the food that has a high level in acrylamide as instant noodles and potato products. LC - UV is also used to analyse residues of acrylamide in polyacrylamide, in the soil or other environmental samples.
In LC-MS/MS by electronebuliseur, cleaning of the aqueous extract using a SPE cartridge is needed to remove interference. Three main peaks are observed : m/z 72 (Molecular ion), m/z 55 (loss of NH3) and m/z 27 (loss of NH3 and C = O). The most intense peak, ion m/z 55, is used for the quantification. For identification, the spectrum of the sample is compared with that of the standard to an energy of ionization of 10eV and 20eV. The LOD can descend up to 3 µg/kg and the LOQ, up to 10 µg/kg. The linear range is 10-10000 µg/kg.
Content submitted to the CC-BY-SA license 3.0. Source : Article Acrylamide from Wikipedia in french (authors)
Recent Posts
Cakes, Candy : Warning on nanoparticles
100 % tested products contained nanoparticles ! [...] Les résultats de cette expérience ont…
How to eliminate toxins ?
After a series of festive meals, caloric menus, alcoholic or carbonated drinks,…
Plastic, not so fantastic!
Plastic pollutes the environment, we all know it. Il faut aussi rappeler que les objets…
How to eat healthy during lunch break ?
At work, lunch breaks are often done on the go, en quelques minutes et…
Eat healthy, it's really possible ?
These last years, le bio et le sain se sont imposés avec insistance dans nos…
Sausages and merguez salmonella
The company L’Atelier du Valois has recalled batches of "Plateau variegated" suite…

